Chlorine corrosion
Understanding chlorine corrosion and how to prevent it
When a fire or other event causes temperatures to rise above about 80° Celsius, products that contain halogens, such as PVC, release chlorine.
Chlorine reacts with moisture in the air to produce aggressive and corrosive hydrochloric acid.
The chemistry behind corrosion
When iron comes into contact with hydrochloric acid, the following reaction occurs:
Fe + 2HCl → FeCl2 + H2
FeCl2 + 2H2O → Fe(OH)2 + 2HCl
4Fe(OH)2 + O2 → 4FeO(OH) + 2H2O
2FeO(OH) → Fe2O3 + H2O
During the corrosion process, hydrochloric acid is released, triggering a new reaction. This is why hydrochloric acid is so aggressively corrosive and causes pitting on stainless steel.
In order to halt the chemical reaction that drives corrosion, you need to isolate one of the four elements – iron, chlorine, hydrogen and oxygen. Since that is not possible with iron (Fe) and oxygen (O), the only way to prevent corrosion is by tackling chlorine (Cl) and moisture (H).
Preventing corrosion
There are three ways to halt corrosion and prevent further damage:
1. Decontamination: This is a special process that uses alkaline chemicals to neutralise the hydrochloric acid and halt the corrosion.
2. Dehumidification: Reducing the relative humidity (rH) by 30–35% ensures that there isn’t sufficient moisture in the air to sustain the chemical reaction, which consequently slows. This is, however, only a temporary solution because as soon as the rH rises above 35%, the corrosion process takes off again.
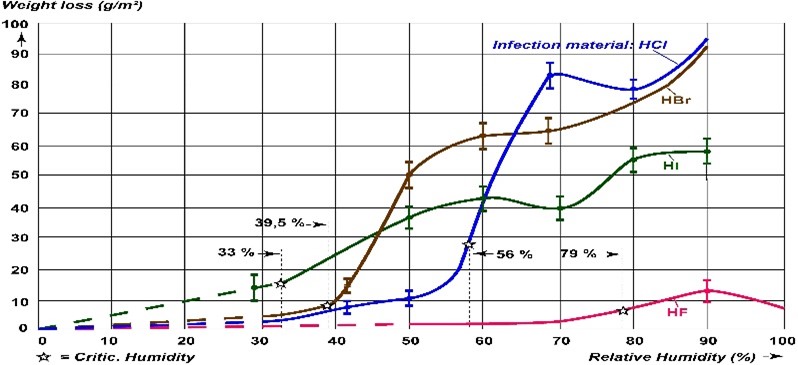
In cases of HCl contamination (blue line), there is a strong correlation between relative humidity (rH) and corrosion.
3. Oiling bare metals: A thin layer of oil prevents moisture in the air from reaching the chlorine, inhibiting the corrosion process for a time. As soon as the layer is damaged or evaporates, the corrosion process will resume.
Since the goal is to put an end to corrosion, the first option is obviously the best choice.
With the other two temporary fixes, corrosion will recur whenever dehumidification ceases or the oil layer breaks down.
Limit values for unprotected metal surfaces in mechanical and electronic systems
Focusing on corporate security and safety, VdS Schadenverhütung GmbH is an association that brings together German property insurers and reinsurers. Together with loss adjusters and other experts, VdS has published guidelines on estimating losses based on levels of corrosive and toxic contamination after (fire) damage.
According to the VdS Guidelines for Fire Loss Restoration 2357 (2007), the critical reference value for corrosion in metals is >10 µg/cm² chlorine (from hydrochloric acid) but 5 µg/cm² for sophisticated electronics and technical equipment.
Limit values for metal surfaces after contamination with hydrochloric acid
| Chlorine concentration | Corrosion risk |
| Under 2 µg/cm² | Non-critical range |
| 2 - 8 µg/cm² | Transitional range |
| Over 8 µg/cm² | Critical range |
Corrosion is contingent on relative humidity (rH) in the areas where the contaminated equipment is located. If the rH is below 30–35% and contamination levels do not exceed 20–30µg/cm², no corrosion will occur. When rH values increase, especially to above 50–60%, metallic surfaces will immediately be subjected to corrosion.

